CEFOVECIN
Category:
Keywords:
CEFOVECIN
Details description
Active Ingredients:
Each 20 mL multi-use vial contains 800 milligrams of cefovecin (in the form of cefovecin sodium) as a lyophilized cake.
Chemical property:
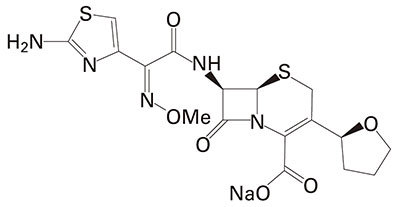
Cefovecin sodium is a semi-synthetic broad-spectrum antibacterial agent from the cephalosporin class of chemotherapeutic agents. Cefovecin is the non-proprietary designation for (6R,7R)-7-[[(2Z)-(2-amino-4-thiazolyl) (methoxyimino)acetyl]amino]-8-oxo-3-[(2S)-tetrahydro-2-furanyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, mono-sodium salt.
Indications:
Cefovecin sodium is indicated for the treatment of skin infections (secondary superficial pyoderma, abscesses, and wounds) in dogs caused by susceptible strains of Staphylococcus intermedius and Streptococcus canis (Group G). Cefovecin sodium is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida. Cefovecin sodium is the first antibiotic for dogs and cats that ensures a course of treatment in a single injection. One dose of Cefovecin sodium provides up to 14 days of safe and effective antibiotic treatment for the most common skin infections. It is well-tolerated, and its safety has been demonstrated in juvenile and adult dogs and cats.
Dosage & Administration:
A sample of the lesion should be obtained for culture and susceptibility testing prior to beginning antimicrobial therapy. Once results become available, continue with appropriate therapy.
Reconstitute Cefovecin sodium with 10 mL sterile water for injection. Shake well until it is totally dissolved with a solution containing cefovecin sodium equivalent to 80 mg/mL.
Dogs
Cefovecin sodium should be administered as a single subcutaneous injection of 3.6 mg/lb (8 mg/kg) body weight. A second subcutaneous injection of 3.6 mg/lb (8 mg/kg) may be administered if response to therapy is not complete. The decision for a second injection for any individual dog should take into consideration such factors as progress toward clinical resolution, the susceptibility of the causative organisms, and the integrity of the dog's host-defense mechanisms. Therapeutic drug concentrations after the first injection are maintained for 7 days for S. intermedius infections and for 14 days for S. canis (Group G) infections. Maximum treatment should not exceed 2 injections.
Cats
Cefovecin sodium should be administered as a single, one-time subcutaneous injection at a dose of 3.6 mg/lb (8 mg/kg) body weight. After an injection of Cefovecin sodium, therapeutic concentrations are maintained for approximately 7 days for Pasteurella multocida infections.
Adverse reaction:
Dogs: Lethargy, anorexia/decreased appeptite, vomiting, diarrhea, blood in feces, dehydration, flatulence, increase borborygmi.
Cats: Vomiting, diarrhea, anorexia/decreased appetite, lethargy, hyper/acting strange, inappropriate urination.
Cautions:
Not for use in humans. Keep it out of reach of children.
Dogs and cats allergic to β-lactam (penicillins and cephalosporins) group are not applicable.
For subcutaneous use in dogs and cats only.
How Supplied:
20 mL multi-use vial containing 800 milligrams of cefovecin
Storage Conditions:
Protect from light, 2° to 8° C.
Dry powder 24 months, reconstituted sterile solution 28 days.
If stored as recommended, solution color does not adversely affect potency.
Product Inquiry